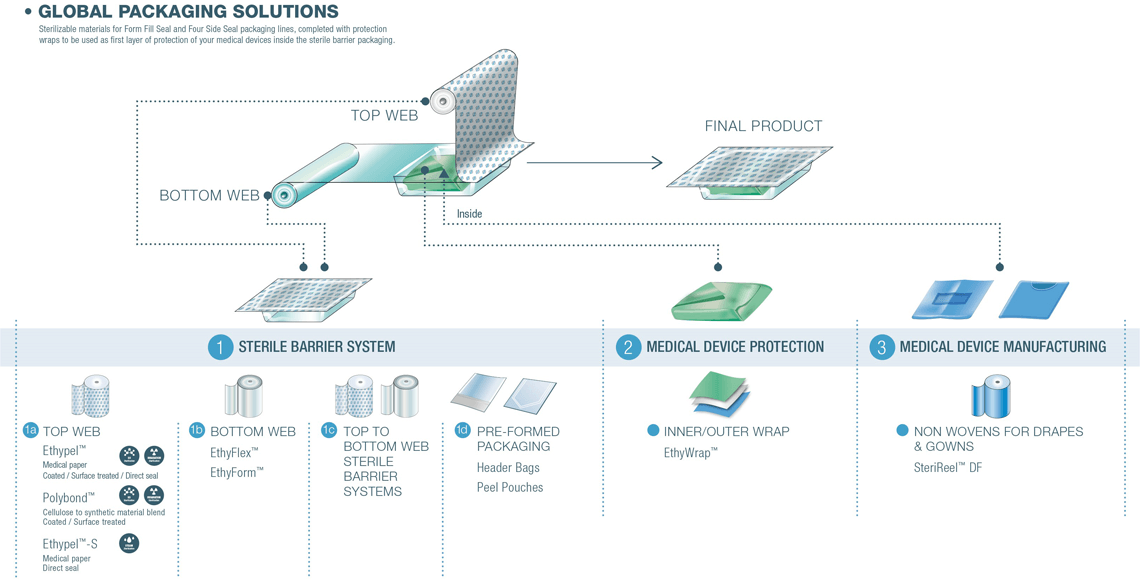
Our global offer
Expertise and Innovative Solutions for Infection Prevention
To best meet the market needs, STERIMED has developed product lines dedicated to different manufacturing and sterilization process types.




 |
Maintaining sterility for 5 years minimum |
FOR:
- Packaging of syringes, catheters, gauzes, drapes, gowns, and many more
- Blister manufacturing
- Coating activities
TECHNICAL CHARACTERISTICS:
- Paper, Film, Reinforced materials
- Coating, Printing, Slitting
- 55 gsm to 117 gsm (34 to 72 lbs.)
- Conforms to EN ISO 11607-1: 2020, EN868-6: 2017, EN868-7: 2017
SEMINARS & TRAINING:
Pack Design Day :
www.packdesignday.com
Read more about Ethypel and Polybond solutions


 |
Maintaining sterility for 180 days minimum |
 |
FOR:
- Manufacturing pouches and tubing for sterilization in patient care units
TECHNICAL CHARACTERISTICS:
- Paper and Reinforced materials
- Printing, Slitting
- 60 gsm to 112 gsm (37 to 69 lbs.)
- Conforms to EN ISO 11607-1:2020, EN868-3: 2017
SEMINARS & TRAINING:
Hospital Pouch Partnership Program:
www.patient-safety-forum.com
Read more about propypel solutions




 |
Maintaining sterility for 90 to 180 days minimum |
FOR:
- Distributors to hospitals
- Healthcare professionals
TECHNICAL CHARACTERISTICS:
- Crepe paper, Reinforced and Non-woven materials from 52 to 78 gsm (32 to 48 lbs.)
- SMS from 40 to 70 gsm (25 to 43 lbs.)
- Filters, Tray liners
- Conforms to EN ISO 11607-1: 2020, EN868-2: 2017
SEMINARS & TRAINING:
Patient Safety Forum :
www.patient-safety-forum.com
Download Sterisheet mobile app at:


